ECT*-LISC researchers develop more precise models to describe DNA damage during radiotherapy
These advances have been recognized by two recent publications in the prestigious journals Journal of Physical Chemistry Letters and Physical Chemistry Chemical Physics. The latter article has been also highlighted in the “2021 PCCP Hot Articles” list as well as in the journal’s back cover.
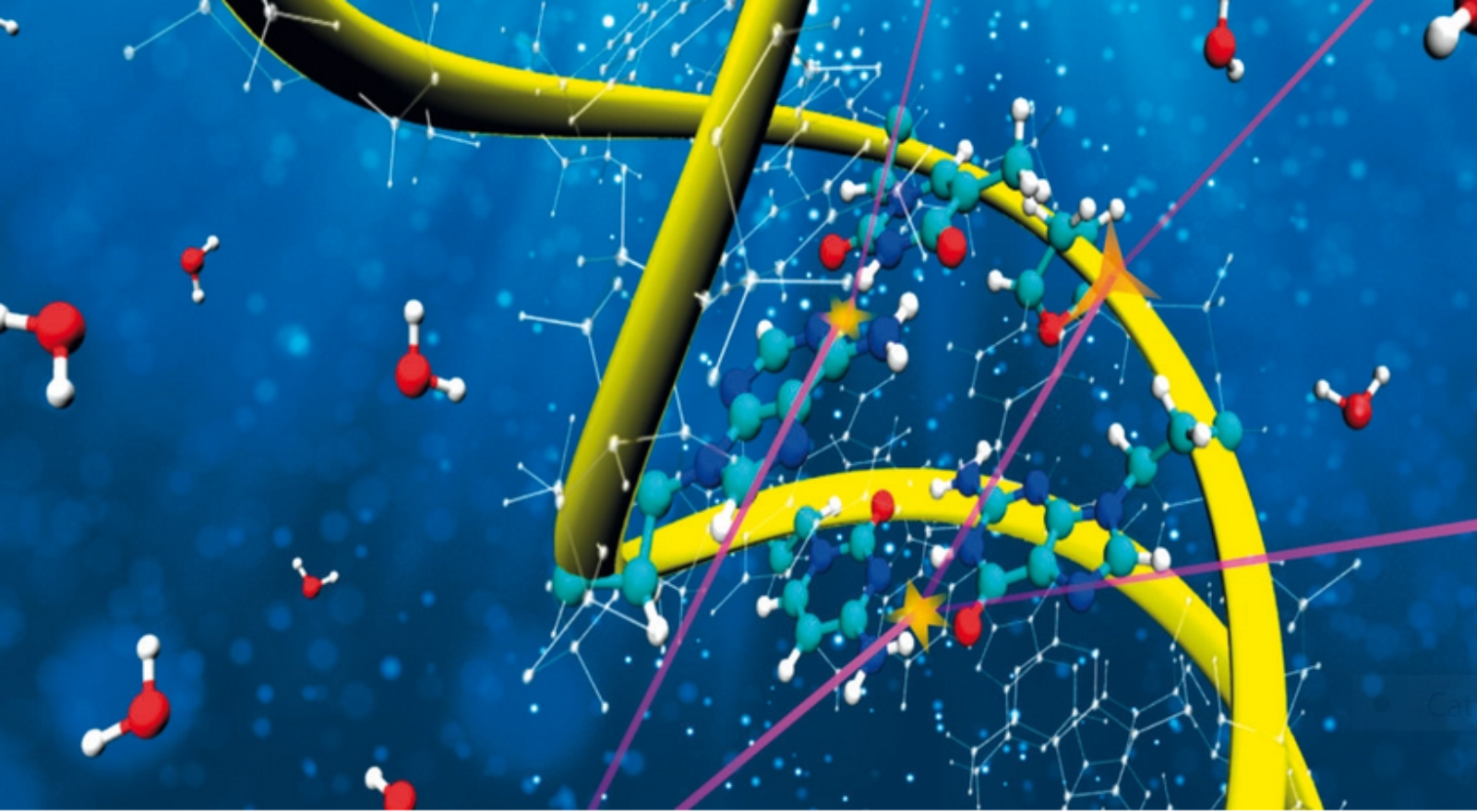
Detailed simulations of complex biodamage during hadrontherapy
In the first investigation, led by Simone Taioli and Maurizio Dapor (Interdisciplinary Laboratory for Computational Science, ECT*-LISC), and published last January by the prestigious Journal of Physical Chemistry Letters, the researchers developed a highly detailed multiscale simulation of the complex nanoscale biodamage produced by carbon ions during hadrontherapy. Advanced quantum mechanical calculations were used to describe the electronic excitations in liquid water with unprecedented precision, and used to obtain reliable interaction probabilities between electrons and the biological medium to be used in Monte Carlo simulations. “It is the first time that the electronic excitation spectrum of liquid water, the main constituent of living cells, has been obtained ab initio in such an excellent agreement with the available experimental findings”, remarks Paolo Trevisanutto (ECT*-LISC), co-author of the article.
Maurizio Dapor highlights one of the most important findings of the study: “In our simulations, we can assess the biodamage on the nanoscale produced by different physical mechanisms. However, experimental setups typically can only measure ionizing collisions. Our study confirms that indeed ionizations account for about 80% of complex biodamage in carbon ion therapy, which supports the use of the widely-used ionization-based experimental nanodosimeters”.
Limitations of current models and a new discovery
However, Monte Carlo simulations currently have certain limitations, as explained by Rafael Garcia-Molina, co-author of the second study: “Generally, Monte Carlo simulations need to approximate the properties of DNA as if it was liquid water, since it is not exactly known how electrons interact with DNA. Although this approach is generally reasonable, water being the most abundant component in biological tissues, a better description of DNA is necessary to make more precise simulations.”
In the second work, led by Pablo de Vera (Marie Curie Individual Fellow at ECT*-LISC), together with Rafael Garcia-Molina (University of Murcia, Spain) and Isabel Abril (University of Alicante, Spain), the researchers have proposed a new theoretical model that allows obtaining the probabilities of electron interaction, in a wide range of energies, with the molecular components of DNA. “The outstanding comparison of our results with an abundant collection of experimental measurements for these relevant biological molecules demonstrates the high predictive capabilities of the new model”, highlights Isabel Abril.
These results are essential to advance the understanding, at the molecular level, of the effects of ionizing radiation on living tissues during radiotherapy, which is necessary to develop more effective treatments. In recognition of this great potential, the journal Physical Chemistry Chemical Physics (PCCP) has included this paper in its “2021 PCCP Hot Articles” list, which highlights those articles considered of special relevance by the journal’s editors and referees. Likewise, this research has also been used to illustrate the back cover of the PCCP journal.
The articles in JPCL and PCCP can be freely downloaded, and a short video about the former publication is available online, see link below.
These investigations are part of the European project NanoEnHanCeMent, led by Pablo de Vera, as well as of the project Nanocater, funded by the Fondazione Caritro of Trento and Rovereto.