Regenerative aging
Experiments by a research group at the University of Galway on some marine organisms allow us to better understand the mechanisms of cellular senescence, and to unveil a number of mysteries about regeneration. The first characteristic anyone associates with the Hydra of Lerna, Hercules' second effort, is surely
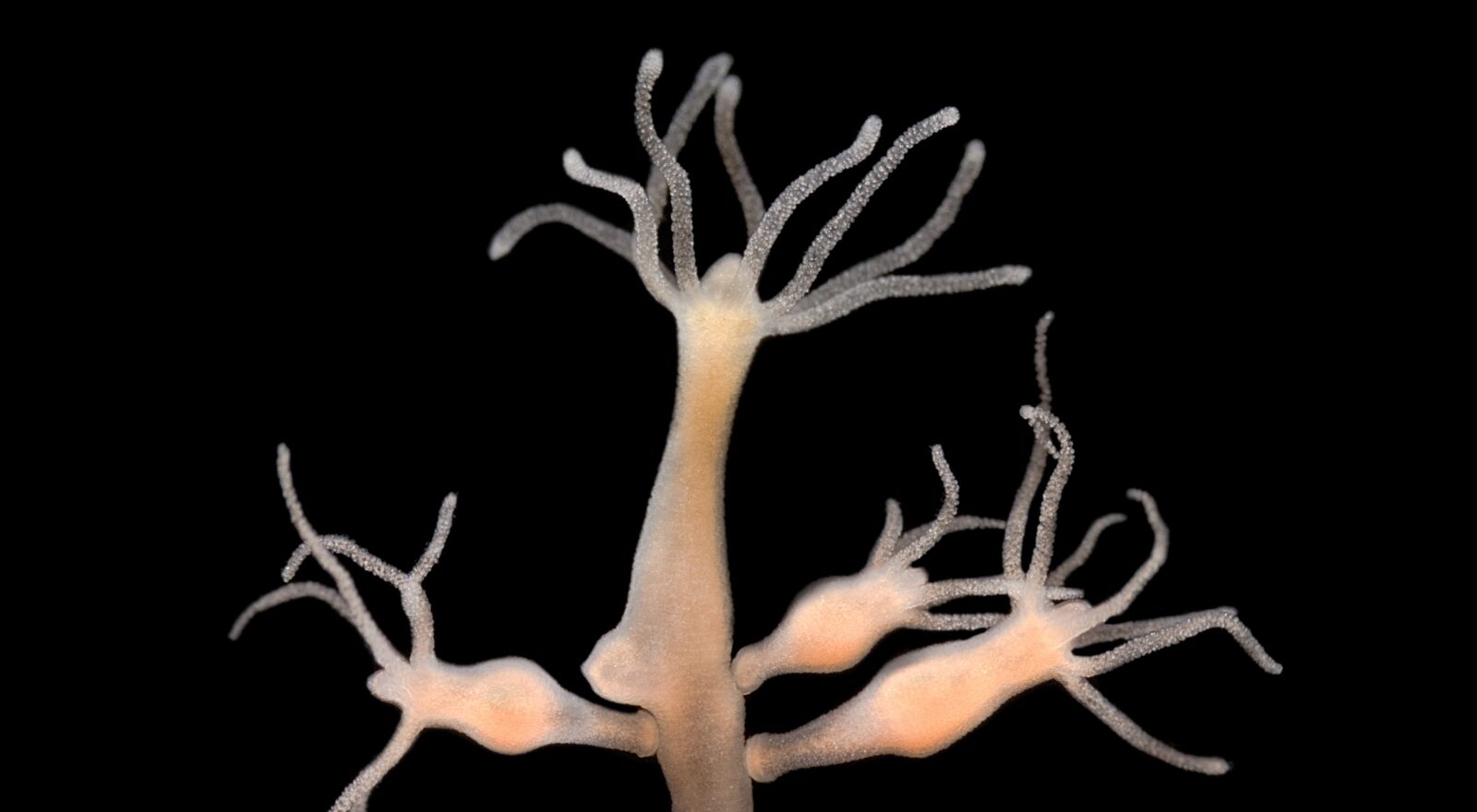
The first characteristic anyone associates with the Hydra of Lerna, Hercules’ second effort, is surely a great regenerative capacity: when deprived of one of its heads, the monster was able to regenerate it (or regenerate two, or three, depending on the version of the myth). However, even beyond human imagination, the natural world is home to even more amazing hydras than the one inhabiting the legend.
Specimens of the species Hydractinia simbiolongicarpus (closely related to the species of the genus Hydra, named after the above monster) if deprived of the head are able to regenerate it completely, but not only that: the head itself is able to regenerate the entire organism. How is this possible?
To answer this question, we first need to identify such organisms within the tree of life. H. simbiolongicarpus belongs to the cnidarian group, the same group that includes jellyfish, corals and anemones of all sorts. In fact, the structure of individuals of this species is very similar to that of a generic anemone: they consist of a hollow stem at the end of which is a head bristling with tentacles.
Professor Uri Frank and colleagues, observing these organisms in laboratories at the University of Galway, Ireland, noticed that the totality of their pluripotent stem cells reside at the base of the stem. It is these cells that, being able to acquire both “structural qualities” and “reproductive qualities,” either cope with the damage the organism suffers, or renew its tissues. It is therefore easy to understand that, following the amputation of the head, the organism manages to regenerate it completely: stem cells migrate from the base of the stem to the injured area and operate regeneration. It is much more complicated to understand, however, how the severed head alone, completely devoid of stem cells, can completely regenerate the body.
Initially, even Prof. Frank and his team were incredulous at the idea that the head of H. simbiolongicarpus was devoid of stem cells, and they conducted several experiments to get the hang of it. Having ascertained the absence of such cells, the researchers found that the only event occurring in the heads of the organisms following amputation, the key to explaining the phenomenon, was the sudden senescence of cells adjacent to the wound. When a cell enters the senescence phase, it acquires a characteristic that is called SASP: Senescence Associated Secretory Phenotype. In other words, senescent cells secrete different molecules than other cells. These compounds are able to “transform” the cells that come into contact with them into stem cells. The severed head of H. simbiolongicarpus is thus filled with stem cells that lead to the regeneration of the organism.
An interesting detail to point out is that the training organism must get rid of senescent cells as soon as possible because, in light of their ability to “transform” neighboring cells, if they remained in the organism for too long they would end up converting more cells into stem cells than necessary, with obvious risks to the animal. For this reason, between 33 and 38 hours after decapitation, the severed head expels all the senescent cells that inhabit it.
The experiments on H. simbiolongicarpus allowed Prof. Frank and colleagues to draw very interesting conclusions: according to them, the appearance of cellular senescence during evolution has allowed organisms with high cellular plasticity to cope with damage of varying intensity. Namely, organisms with easily “transformable” cells have used, and use, senescence as a trigger for the regeneration mechanisms that are carried out by stem cells. Unfortunately, however, for organisms with low cellular plasticity, such as H. sapiens, this mechanism is not as useful because it would undermine the stability of the structures that ensure their survival. Nevertheless, the experiments of Prof. Frank and his team offer our species the opportunity to understand more deeply the secrets of cellular senescence, which, can be very useful at a time when the population of the Western world is aging more and more.